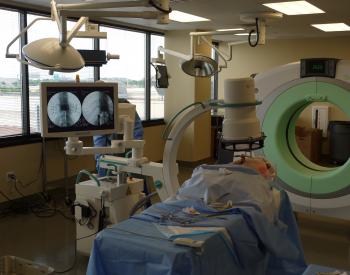
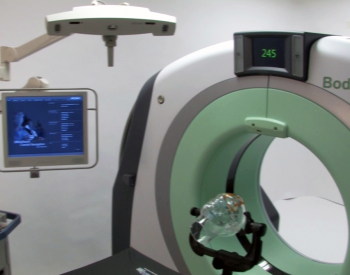
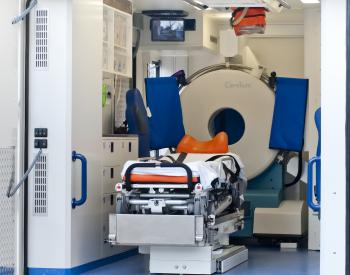
CT images deemed compatible with robotic surgical technology
Danvers, Ma (July 1st, 2012) – NeuroLogica Corporation, the developer of cutting edge portable imaging devices, today announced the successful validation of the images provided by its portable 32 slice CT scanner, BodyTomTM for use with Mazor Robotics RenaissanceTM surgical guidance robot.
Mazor Robotics RenaissanceTM robot executes highly accurate, complex spinal procedures delivering increase patient safety, fewer complications and requiring lower radiation doses as compared to traditional surgical intervention. Utilizing the images from a high quality pre-operative CT scan, attending surgeons carefully map out the procedure in a three-dimensional, virtual environment ensuring accurate placement of screws and implants. The images provided by NeuroLogica’s portable CT scanner BodyTomTM were proven to be compatible with Mazor’s planning software.
“We are pleased to announce the successful validation between the BodyTom and the Renaissance robot,” says Dr. Eric M. Bailey, CEO of NeuroLogica. “Soon patients across the globe will benefit from the combination of the superior images of BodyTom and the cutting edge technology developed by Mazor Robotics.”
“Compatibility of Mazor’s Renaissance robot with leading imaging products is important to our customers” said Ori Hadomi, CEO of Mazor Robotics. “So while surgeons can still use regular CT scanners for the planning, they can also use intra-operative CTs, such as BodyTom, which are quickly gaining popularity with them.”
NeuroLogica’s BodyTom™ is a completely portable, full body 32 slice CT that boasts an 85cm gantry and 60cm field of view. The battery powered BodyTom™ can be transported from room to room, similar to the widely used portable chest X-ray systems. Its unique capabilities provide high quality CT images wherever needed, including the clinic, ICU, OR and Emergency/Trauma Department. BodyTom™ is DICOM 3.1 compliant and compatible with all PACS, surgical navigation, electronic medical records, and planning systems.
For more information on the successful validation between NeuroLogica’s BodyTomTM and Mazor Robotics RenaissanceTM robot, please contact Jason Wheeler at 978-564-8576 or by email at jwheeler@neurologica.com.
About NeuroLogica Corporation
Located in Danvers, Massachusetts, NeuroLogica Corporation (www.neurologica.com) specializes in the design and manufacture of cutting edge imaging equipment that is easy to use and brings the power of imaging to the patient. NeuroLogica`s BodyTom™ CT and CereTom® CT are FDA registered and the quality system is certified to ISO 13485:2003 and ISO 9001:2008 with Canadian Medical Device Amendments. NeuroLogica`s BodyTom™ CT and CereTom® CT are CE marked (European Conformity) for distribution in the European Union and European Economic Area.