Featured product debuts include the NExCT 7 CT scanner and the RS80A with Prestige ultrasound system
“Through our innovative designs and advanced technologies, we remain committed to delivering new solutions, like the NExCT 7 and the RS80A with Prestige, that unlock workflow efficiencies for medical professionals as well as improve patients’ lives,” said Soo-In Cho, President and Head of the Health & Medical Equipment Business at Samsung Electronics Co., Ltd. “By leveraging the combination of Samsung imaging technology and user-inspired design, we will continue to deliver on our promise of fast, easy and accurate diagnostic solutions for our customers in the healthcare community.”
Computed Tomography (CT) Segment
Samsung’s CT imaging solutions are used throughout the world in intensive care units, neurosurgical operating rooms, emergency departments, stroke centers and other key clinical areas. Debuting at RSNA is:
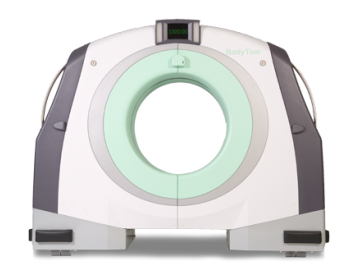
- NExCT 7 – the first premium CT scanner from Samsung features high resolution designed to improve diagnostic confidence in advanced and routine exams reducing the radiation dose as low as reasonably achievable and ensuring a fast and efficient workflow. Some features of the NExCT7 are FDA 510(k) pending.
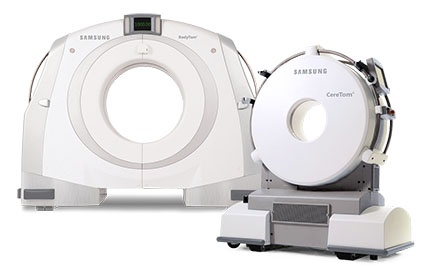
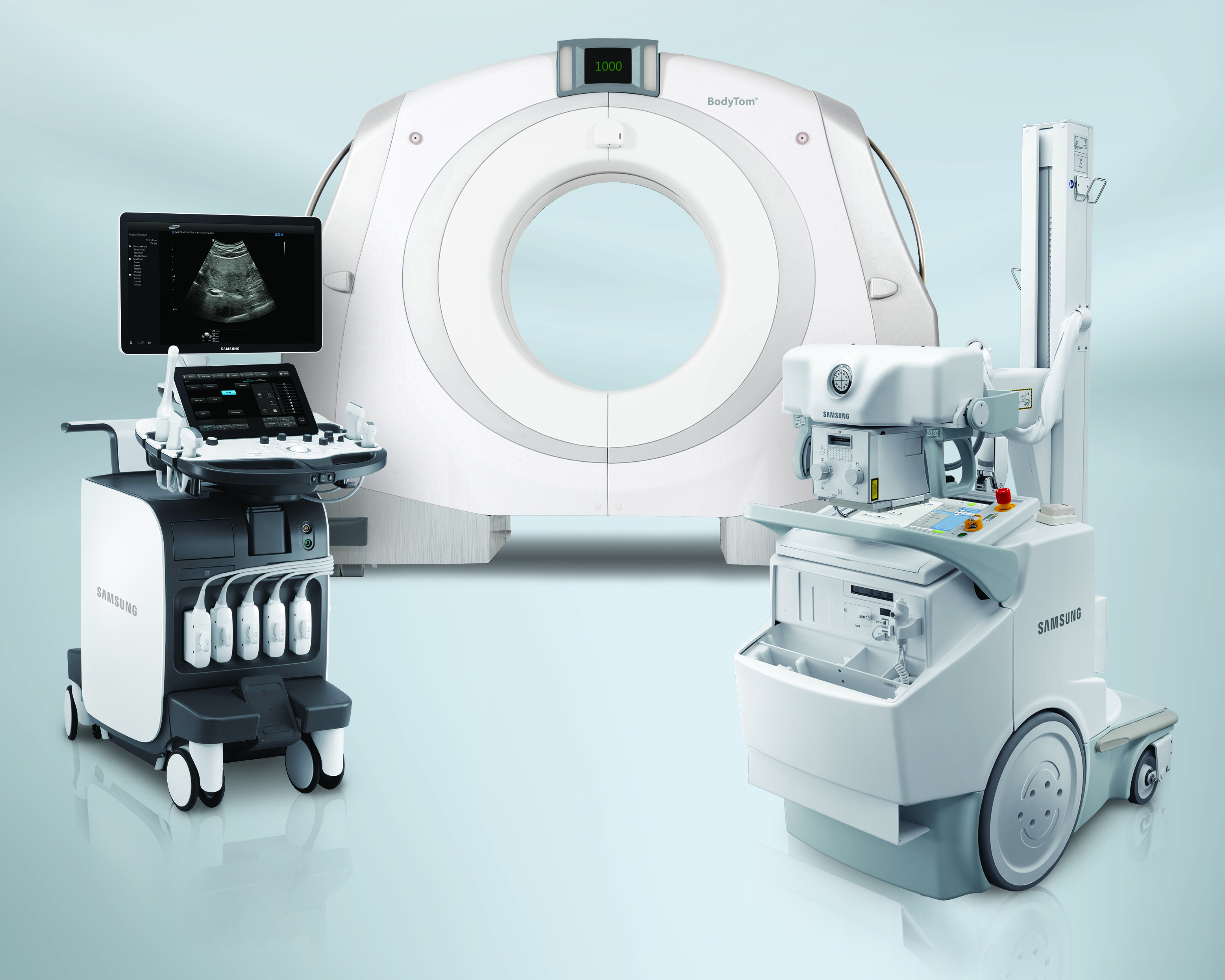
- Portable CT – Samsung will be showcasing the latest in software and hardware enhancements across its portable CT product line. These upgrades improve user satisfaction and streamline workflow, and both systems are now compliant with the Smart Dose (XR-29) radiation dose reporting standard.
The BodyTom and CereTom portable systems continue to prove their versatility as they are increasingly adopted and utilized in a wide range of market applications such as mobile stroke units, brachytherapy suites, proton therapy centers, deep brain stimulation and even veterinarian hospitals.
Ultrasound Segment
Samsung continues to enhance the user experience with its cutting-edge diagnostic ultrasound devices around the world and across various medical fields. On display at RSNA are:
- RS80A with Prestige – now available in the U.S. market, this system features advanced technical capabilities built on the successes of Samsung technologies. Product features include:
- S-Fusion to enable simultaneous localization of lesions with a real-time ultrasound image supported by other modalities' 3D data sets. Fusion speed and accuracy are the strengths of S-Fusion.
- S-Shearwave to detect the velocity of the shearwave transmitted through the targeted lesion and display the numerical measurement of stiffness.
- E-Breast*: this is the function for breast lesion exam, which calculates the strain between the area of the suspected lesion (ROI) and normal breast fat and displays the results.
- E-Thyroid*: This function assists with the diagnosis of thyroid lesions. The ROI is used to show the contrast of an elastogram.
*The strain ratio value is a relative value regarding quantification, not an absolute value.
- HS70A – designed for hospital and private care. This system has a flexible architecture to meet the clinical needs of varied users. The HS70A offers a complete Radiology and Cardiovascular solution, including technologies migrated from the premium radiology RS80A platform. Features include S-Detect, which uses Breast Imaging-Reporting, and Data System (BI-RADS®) scores for analysis and classification of suspicious lesions.
Digital Radiography (DR) Segment
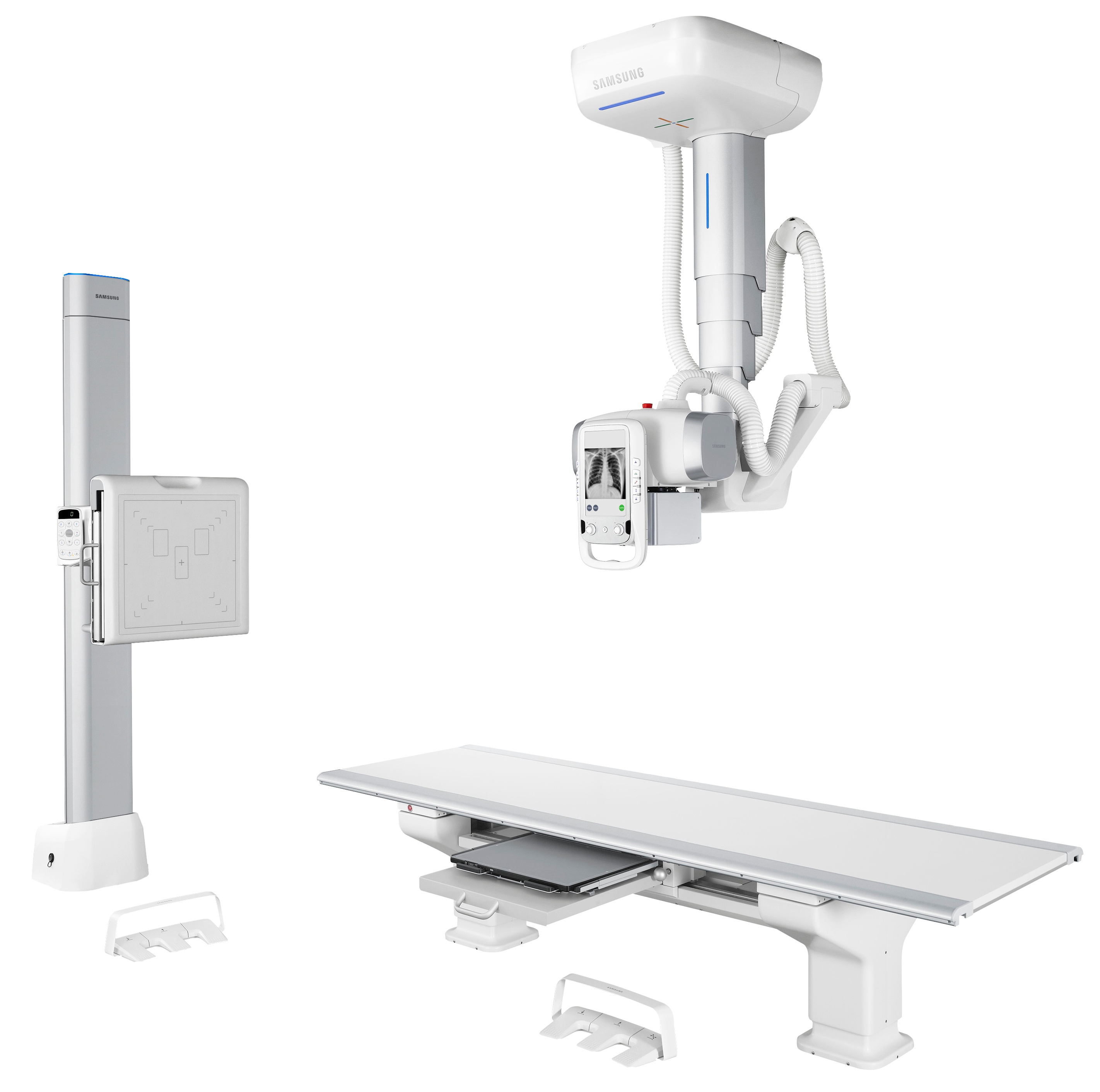
Samsung’s DR systems combine advanced imaging technology, award-winning design and user-friendly automated workflows to meet the demands of 21st century radiology departments. Products on display at RSNA include:
- GC85A – a DR system offering streamlined workflow, diagnostic confidence and improved total cost of ownership. The GC85A features imaging engine S-Vue, ensuring consistency with high quality, convenient setting of contrast and improved sharpness and clarity, S-Share to support unique clinical needs and continuous care with increased connectivity, and Smart Control for one-touch precise positioning.
- GM60A – a mobile Digital Radiography system that delivers high-quality imaging directly to the patient’s bedside. Advanced mobility allows use in the patient room, emergency room, operating room and the intensive care unit, and has advanced features, including S-Detector and S-Vue imaging technologies to deliver accurate diagnoses while ensuring reliable operation with enhanced usability. Additionally, the telescopic column ensures fast navigation by securing a clear view for users.
For more information about Samsung’s showcase at RSNA, please visit http://www.samsung.com/rsna2015. To learn more about Samsung’s healthcare work, please visit samsung.com/global/business/healthcare/.
To read this press release on Business Wire, please click here.
About Samsung Electronics Co., Ltd.
Samsung Electronics Co., Ltd. inspires the world and shapes the future with transformative ideas and technologies that redefine the worlds of TVs, smartphones, wearable devices, tablets, cameras, digital appliances, printers, medical equipment, network systems, and semiconductor and LED solutions. We are also leading in the Internet of Things space with the open platform SmartThings, our broad range of smart devices, and through proactive cross-industry collaboration. We employ 319,000 people across 84 countries with annual sales of US $196 billion. To discover more, and for the latest news, feature articles and press material, please visit the Samsung Newsroom at news.samsung.com.
About NeuroLogica
NeuroLogica, a healthcare subsidiary of Samsung Electronics Co., Ltd., develops, manufactures, and markets innovative imaging technologies and is committed to delivering fast, easy and accurate diagnostic solutions to healthcare providers. NeuroLogica, the global corporate headquarters and manufacturer of computed tomography, is also the U.S. headquarters for sales, marketing and distribution of all Samsung digital radiography and ultrasound systems. NeuroLogica’s growing portfolio of advanced medical technologies are used worldwide in leading healthcare institutions helping providers enhance patient care, improve patient satisfaction, and increase workflow efficiency. Samsung is committed to being leaders in the field of healthcare imaging. For more information, please visit www.SamsungNeuroLogica.com.




 From the April 2015 issue of Columbus CEO — Interventional radiologists have emerged from X-ray labs into operating rooms, using minimally invasive techniques to treat hard-to-reach parts of the body and turning a low-profile specialty into a burgeoning surgical field with its own clinical and outpatient services. In the process, they're reducing costs, shortening recovery times and easing pain.
From the April 2015 issue of Columbus CEO — Interventional radiologists have emerged from X-ray labs into operating rooms, using minimally invasive techniques to treat hard-to-reach parts of the body and turning a low-profile specialty into a burgeoning surgical field with its own clinical and outpatient services. In the process, they're reducing costs, shortening recovery times and easing pain. February 11, 2015 — Patients treated with thrombolysis in a
February 11, 2015 — Patients treated with thrombolysis in a  February 12, 2015
February 12, 2015