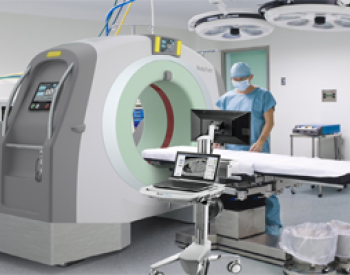
Navigation-guided procedure has been widely conducted in the neurosurgical filed to perform anatomy-oriented approach. However, the approach cannot reflect the real-time intracranial image due to differential spatial displacement caused by cerebrospinal fluid drainage, volume change after surgical evacuation or newly developed lesions such as
Press Releases
Danvers, MA (February 25, 2013) – NeuroLogica Corporation, a subsidiary of Samsung Electronics, has named Michael Limoli as the Vice President of Engineering. Limoli will oversee day-to-day engineering operations and facilitate engineering product development.
Limoli began his career with NeuroLogica in 2004 as the Principal Software
Ridgefield Park, NJ – January 28, 2013 – Samsung Electronics America, Inc., a subsidiary of Samsung Electronics Co. Ltd, today announced its acquisition of NeuroLogica, a leading Computed Tomography (CT) company headquartered in Danvers, Massachusetts. Established in 2004, NeuroLogica develops cutting-edge medical imaging
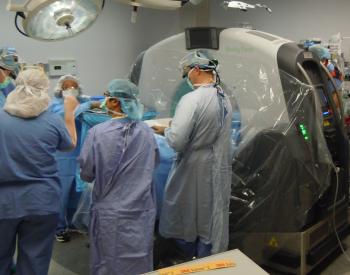
In an Australian first, patients at a Brisbane hospital will no longer need to be transported out of the operating theatre so that surgeons can do vital imaging crucial in the outcome of their surgery.
This has been made possible through a unique fully portable CT scanner that has been installed in Brisbane Private Hospital through the Australian
Nashville, TN and Danvers, MA – Pathfinder Therapeutics, the developer of computer-assisted surgical navigation products for soft tissue organs, and NeuroLogica Corp., the manufacturer of portable CT imaging devices, announced today an agreement to integrate their innovative technologies.
In use at top cancer centers in the US
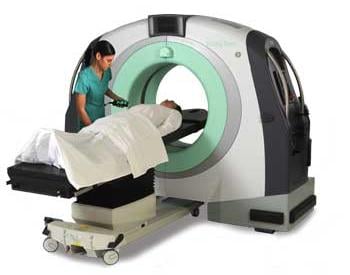
WARREN, MI - Providence Hospital is one of the first in the region to offer a unique portable, full body CT scanner.
Known as the BodyTom, the battery operated, movable intra-operative 32 slice CT scanner provides surgeons with new capabilities during procedures. The BodyTom is the first scanner of its kind, allowing imaging
Danvers, MA (October 16, 2012) - STERIS Corporation (NYSE: STE), a recognized leader in surgical support and integration technologies, and NeuroLogica Corp., designers and manufactures of portable imaging equipment, have formed a collaborative relationship to offer fully integrated, intraoperative surgical suites to healthcare systems. The goal of
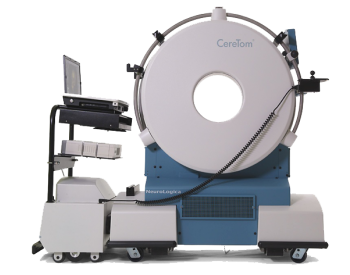
Danvers, MA (August 1st, 2012) – NeuroLogica Corporation, a medical device company, announced today that it has received approval from the Mexican Ministry of Health’s Comisi?n Federal para la Protecci?n contra Riesgos Sanitarios, COFEPRIS, for CereTom®, a portable multi-slice CT scanner for the head and neck.
DANVERS, MA (July 12th, 2012) - NeuroLogica is pleased to announce the integration of NeuroLogica’s portable BodyTomTM 32-slice CT scanner with Medtronic’s StealthStation® S7® surgical navigation system.
This collaboration enables NeuroLogica’s portable BodyTom CT scanner and Medtronic’s StealthStation® surgical navigation system to be used in cranial neurosurgery procedures through the use of Medtronic’s Automatic Intraoperative Registration (StealthAiR™) technology. StealthAiR™ is a fully automatic, universally compatible, patient registration process for sites using intraoperative CT scanners and Navigation.
"NeuroLogica is pleased to announce this integration with Medtronic,” said Dr. Eric Bailey, President and CEO of NeuroLogica. “As intraoperative imaging becomes a staple in the OR, the integration between BodyTomTM and StealthAIRTM will serve to enhance patient outcomes and stretch the limits of what was previously thought possible in complex neurosurgical cases."
NeuroLogica’s BodyTom™ is a completely portable, full body, 32 slice CT that boasts an impressive 85cm gantry and 60cm field of view (FOV). The battery powered BodyTom™ can be transported from room to room and its unique capabilities provide high quality CT images wherever needed, including the clinic, ICU, OR and Emergency/Trauma Department. BodyTom™ is DICOM 3.1 compliant and compatible with all PACS, surgical navigation, electronic medical records, and planning systems.
For more information on the integration between Medtronic’s StealthAiR™ and NeuroLogica’s BodyTomTM, please contact Jason Wheeler at 207-251-2347 or by email at jwheeler@neurologica.com.
About NeuroLogica Corporation
Located in Danvers, Massachusetts, NeuroLogica Corporation (www.neurologica.com) specializes in the design and manufacture of cutting edge imaging equipment that is easy to use and brings the power of imaging to the patient. NeuroLogica`s BodyTom™ CT and CereTom® CT are FDA registered and the quality system is certified to ISO 13485:2003 and ISO 9001:2008 with Canadian Medical Device Amendments. NeuroLogica`s BodyTom™ CT and CereTom® CT are CE marked (European Conformity) for distribution in the European Union and European Economic Area.
Corporation to Optimize Thoracic and Abdominal Interventions in a Standard Operating Room
Wakefield, MA (July 9th, 2012) – ActiViews, Inc, a company pioneering the development of simple, accurate, and cost-effective surgical navigation solutions, announced a partnership today with NeuroLogica Corporation to develop an integrated surgical navigation system compatible with BodyTom™, the portable, full body 32-slice interoperative CT scanner.
The ActiViews CT-Guide™ navigation system is designed to assist physicians during CT-guided interventional, diagnostic and therapeutic procedures such as precutaneous biopsies, ablations and marker placements in the lung and liver. The system shows surgeons the real-time location of their interventional instruments and the trajectory to target on the CT images like a GPS guides a car using stored roadmaps.
“We are pleased to announce our partnership with NeuroLogica,” commented Christopher von Jako, President of ActiViews, Inc. “The combination of our patented CT-Guide™ navigation technology platform with their exclusive, portable, self-shielded CT scanner will offer surgeons a unique enabling technology for minimally invasive thoracic and abdominal oncology procedures in a standard operating room.”
NeuroLogica’s BodyTom™ is a completely portable, full body, 32-slice CT scanner that boasts an 85cm gantry and 60cm field of view. The battery powered BodyTom™ can be transported from room to room, instantly transforming any OR, ICU or trauma bay into a fully-functioning, high quality CT imaging suite. BodyTom™ is DICOM 3.1 compliant and compatible with all PACS systems.
“We are excited to be partnered with ActiViews and their game changing navigation technology,” commented David Webster, Global Vice President of Sales and Marketing. “We believe that BodyTom™ will accelerate the adoption of minimal invasive thoracic and abdominal oncology procedures in a standard operating room.”
About ActiViews:
ActiViews (www.activiews.com) is a privately held high-tech medical device company that develops, manufactures, and markets novel surgical navigation solutions. The company’s mission is to become the leading provider of simple, accurate, and affordable navigation solutions in order to enhance physician confidence in performing minimally invasive thoracic and abdominal surgical oncology procedures. The U.S. Patent and Trademark Office has approved the core technology platform patent with other ancillary patents currently pending. CT-Guide™ navigation is the first and only commercially available system for CT-guided interventions cleared for both lung and liver applications. ActiViews, Inc. is located in the metro-Boston area with Research and Development facilities in Haifa, Israel. ActiViews, Navigate with Confidence™.
About NeuroLogica Corporation:
Located in Danvers, Massachusetts, NeuroLogica Corporation (www.neurologica.com) specializes in the design and manufacture of cutting edge imaging equipment that is easy to use and brings the power of imaging to the patient. NeuroLogica`s BodyTom™ CT and CereTom® CT are FDA cleared and certified to ISO 13485:2003 and ISO 9001:2008 with Canadian Medical Device Amendments. NeuroLogica`s BodyTom™ CT and CereTom® CT are CE marked (European Conformity) for distribution in the European Union and European Economic Area.